Aseptika Ltd (Activ8rlives) are attending Arab Health and are looking to expand efforts in establishing collaborations with clinicians and hospitals specialising in childhood asthma, seeking investment and a manufacturing base in the United Arab Emirates and Saudi Arabia.
Research from the Global Asthma Network, points to approximately 14% of the UAE population are impacted by asthma, equating to 1.3 million people, well above the global average because of the dusty conditions and seasonal changes. From the UAE and Saudi Arabia, we have had significant interest in the early results from the Randomised Controlled Trial of our Asthma⁺me solution for children aged 6-12 years with moderate-to-severe asthma. For further information, come and visit our Stand H2-G50 to discuss the Asthma⁺me solution and learn how it can be translated into your language with a local educational syllabus.
Asthma⁺me was co-designed with a Paediatrician at an NHS Trust (UK) supporting the transition from treatment in specialist paediatric clinics to community care, with an initial focus on meeting the needs of children aged 6-12 years with moderate-to-severe asthma. It supports wireless medical monitors, medication diaries, trigger alerts for pollution/pollen/weather, symptoms tracking, care plan, an extensive educational syllabus and weekly reports going directly to their hospital records.
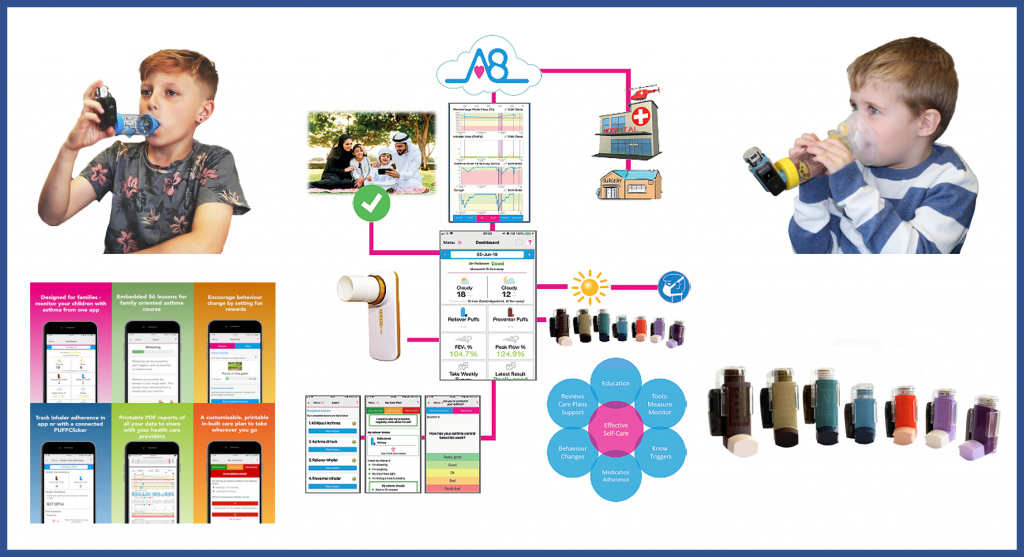

The Asthma⁺me App is used with the Activ8rlives PUFFClickerᵀᴹ smart tracker for pressurised metered-dose inhalers pMDIs, counting doses, recording inhaler shaken properly and timer supporting improved inhaler technique. Asthma⁺me provides basic disease monitoring via connected devices, patient and parent/carer education, medication adherence, motivational support through self-care to reduce severity of asthma attacks, reducing A&E and hospital admissions.
Children are involved in setting their goals and are motivated by incentives from parent/carer to promote behaviour change, increasing adherence to their Care Plan, which is generated automatically on their Smartphone.
What is unique about it?
Current development and integration of AI components during the RCT will significantly enhance the solution allowing disease status reporting to the specialist clinic, less clinician time and in-built expertise for patients and parents. The Asthma⁺me App is the first remote monitoring self-management solution in paediatric clinical trials, that allows risk stratification on need, a personalised-care approach, thereby reducing emergency admissions, improving patient safety and quality-of-life.
How does it benefit clinicians and patients?
Lack of medication adherence is a global problem, with £300million worth of medications/year wasted in primary care, with 50% of patients are not taking their medication as prescribed. Asthma is the most common chronic medical condition among UK Children/Young People. The cost associated with treating all types of asthma is significant. In the UK 10% of children with asthma are clinically-defined as moderate-to-severe or difficult-to-treat asthma and should be treated in specialist paediatric hospital clinics. There are no current comparators to Asthma⁺me worldwide.
What healthcare system need does your product meet?
Reducing Outpatient Appointments at tertiary hospital clinics for moderate-to-severe asthmatic children 6-12 years, so that more first appointments can be provided to other children. It also assists with medication adherence, education, improved self-confidence in self-care of childhood asthma, reduction of admissions and improved use of NHS resources.
Health economics
Assuming the average cost per family of each child with moderate-to-severe asthma treated as Out-Patient is £5,000-6,000pa in the UK. The intervention would need to provide 13.3% reduction in costs to be cash-neutral in Year 1. This does not include the costs associated with lost work-school days, reduced CO2 emissions or benefit of reducing waiting times for first appointments at paediatric clinics. As part of the Asthma⁺me RCT a health economics study will be undertaken to look at the financial and health impacts of this innovative Asthma⁺me care pathway.
Activ8rlives Asthma⁺me solution generates huge amounts of data, making it challenging to review all the patient data. Internal development programmes have demonstrated the use of Deep Learning/Neural Networks (Artificial Intelligence) that can be used to accurately forewarn of decline in health by up to 7-days and providing patients and parents/carers with actionable insights with enough time to start emergency treatment with antibiotics or steroids for example.
-End-
Further information:
Activ8rlives, Activ8rlives.com, Asthma⁺me and Active⁺me are trademarks of Aseptika Ltd.
For further details, please contact Jessica Auton on +44 (0)1480 352 821 or email jessica.auton@aseptika.com
For a case study of Asthma⁺me follow this link
Aseptika Ltd www.activ8rlives.com
Aseptika Ltd began developing Activ8rlives in 2010 and is currently developing its fourth generation of integrated systems, which can be used by consumers and their healthcare service providers using a wide range of platforms or devices to better enable effective and easy self-monitoring.
Incorporating sensors and monitors ranging from consumer accessories to in vitro diagnostics (IVDs). Our focus is: respiratory and cardiovascular disease, cancer, promoting physical activity and weight management. Aseptika Limited has been certified by BSI to ISO 13485:2016 under certificate number MD691414.
(V1.0) Final
