Pulse Oximeter3
£69.99 £58.32

BuddyBand2
£59.99 £49.99

SmartOne Peak Flow & FEV₁ Meter
£99.99 £83.32
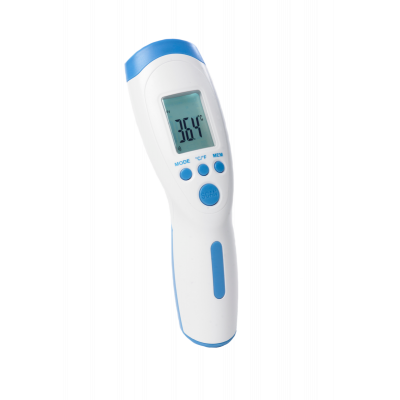
Contactless Thermometer
£49.99 £41.66

Activ8rlives PUFFClicker Smart pMD Inhaler Tracker
£98.50 £82.08
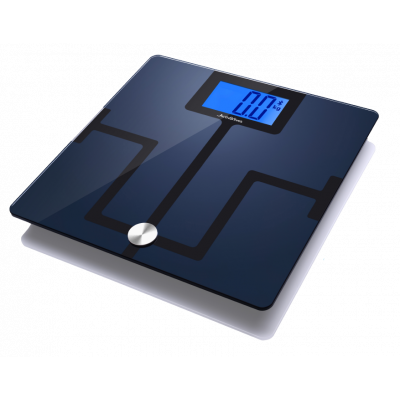
Body Analyser Smart Scales
£49.99 £41.66

Activ8rlives Blood Pressure3 Monitor
£69.99 £58.32